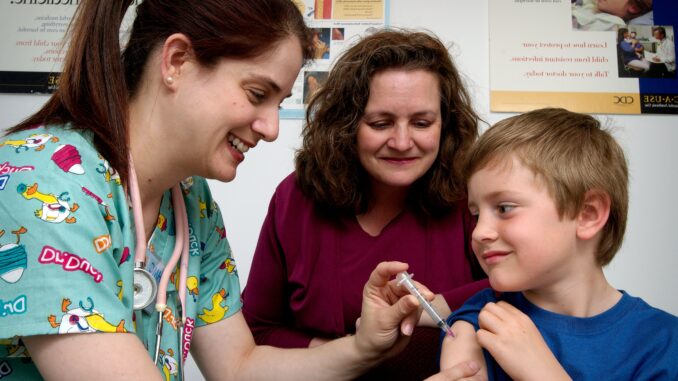
As of Oct. 29 the Food and Drug Administration (FDA) has authorized the emergency use of the Pfizer-BioNTech COVID-19 vaccine for children between the ages of five and 11.
“The vaccine’s safety was studied in approximately 3,100 children age 5 through 11 who received the vaccine and no serious side effects have been detected in the ongoing study,” reads the FDA’s news release on the topic of vaccines for children.
This ongoing study resulted in the FDA’s decision to authorize the use of the vaccine on these younger children. The study is being conducted in multiple countries including the United States, Finland, Poland and Spain.
The FDA looked at the immune response of participants within the five to 11 year old age group along with the immune response of participants in the 16 to 25 year old age group from a previous study and found that the immune responses were close enough to authorize the Pfizer vaccine for use in this younger age group.
“Immune responses of children 5 through 11 years of age were comparable to those of individuals 16 through 25 years of age,” said the FDA. “In addition, the vaccine was found to be 90.7% effective in preventing COVID-19 in children 5 through 11.”
The vaccine for young children is slightly different compared to the version given to adults. The five to 11 vaccine holds a 10 microgram dosage while the 12 and older vaccine is a 30 microgram dosage. The 10 microgram version is administered twice with three weeks in between each dose, much like the vaccine for older individuals.
“It would be a good idea to vaccinate your kids,” said Chief Medical Advisor to President Biden, Dr. Anthony Fauci to CNN’s Don Lemon. “From a statistical standpoint when children get infected it’s much more likely that they would not have a severe outcome like an elderly person would, but that doesn’t mean the children are exempt from a serious illness.”
The country has been on a downward trend in regards to COVID-19 cases lately and the news of the vaccine becoming available to younger children will work to lower the overall case numbers nationwide and locally.
The current number of active COVID-19 cases in Ulster county as of Oct. 29 is 284. Compared to early September’s 500 to 600 active case number, the trend is decreasing given that the vaccine has been authorized for such a young group of people. Parents in Ulster County should feel safer knowing that their kids can be vaccinated and even more protected from COVID-19 than before, as seen in the Ulster County COVID-19 dashboard.
While the current FDA regulation regarding COVID-19 vaccines for children aged five to 11 is just an emergency authorization, the FDA does feel strongly that the lower dose vaccine is safe for children to take and will benefit everyone in the long run.
Next week, the Center for Disease Control’s (CDC) Advisory Committee on Immunization Practice will meet to discuss further recommendations. The CDC and FDA work together to monitor the state of the COVID-19 pandemic and work to make the best choices for all people to protect public health.

